Evidence-based medicine incorporates three principles1-2.
1. A health care provider’s clinical expertise
2. Best available external clinical evidence from systematic research
3. Patient values and expectations
What is the hierarchy of research studies suggesting the order of scientific reliability?
The hierarchy of research studies reflecting the order of scientific reliability is generally viewed as follows from the top as the most reliable and then down each level to the bottom.
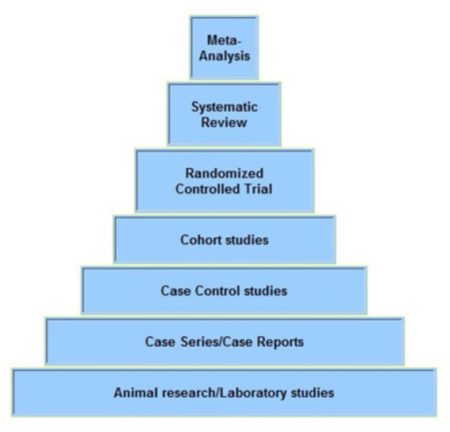
The evidence pyramid ranks the available literature in a hierarchy and how it evolves. The best level of evidence to answer scientific questions does not always exist. Less published literature is available from the bottom to the top of the pyramid. Going down the pyramid to other types of studies can be necessary.
The pyramid base begins with a conceptual idea. When experiments support further investigation into drugs and even diagnostic tools, tests with enough evidence are done in lab models, animals, and humans. Research tests in humans are performed in cancer clinical trials evaluating scientific questions with emphasis on safety and efficacy.
Meta-Analysis
Examines and analyzes many studies for a specific topic. Results are combined using statistical methodology and with analysis of randomized controlled trials.
Systematic Reviews
Focuses on a clinical topic related to cancer and answers a question. A detailed literature search selects all studies with high quality methodology. Studies are reviewed, assessed, and the results summarized.
Randomized Controlled Clinical Trials
Carefully planned projects that study the effect of a therapy on people. They include methodologies that reduce the potential for bias (randomization and blinding) and that allow for comparison between intervention groups and control groups (no intervention).
Studies that show the efficacy of a diagnostic test are called prospective, blind comparison to a gold standard study. This is a controlled trial that looks at patients with a specific type of illness.
Cohort Studies
Takes a large population and follows patients who have a specific condition or receive a particular treatment over time. They are compared with another group that has not been affected by the condition or treatment being studied. Cohort studies are observational and not as reliable as randomized controlled studies since the two groups may differ in ways other than in the variable under study.
Case Control Studies
Studies where patients with a specific condition are compared to people without it. Medical records and data recall by patients are collected. Case control studies are perceived as less reliable in comparison with randomized controlled trials and cohort studies. A statistical relationship does not confirm that one factor caused the other.
Case Series and Case Reports
Includes reports on treatments to small groups or one patient. Reports of a case or cases use no scientific control groups to compare outcomes. Case series and case reports are viewed without validity statistically.
What is the process for translating research results into clinical practice and patient care?
From a presentation by R. Brian Haynes, MD, PhD entitled Evidence-Based Information Resources through the Centre for Evidence-Based Medicine, the graphic describes the evolution of information resources for evidence-based medicine.
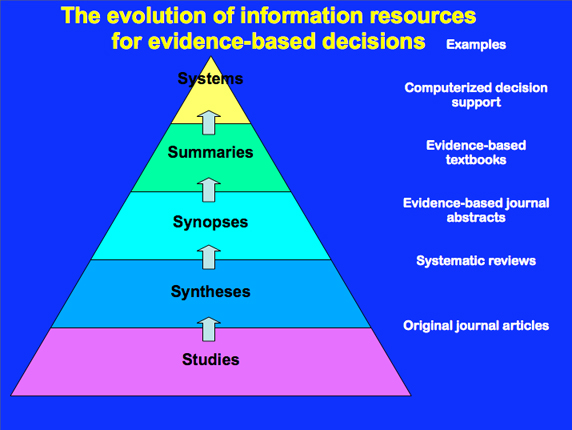
Research results of cancer studies may or may not be published in scientific journals.
Published randomized clinical trials may or may not be referenced in systematic reviews.
Studies may or may not be included in evidence-based journal abstracts, evidence based textbooks, and computerized decision support.
Therefore, study results are not always translated into clinical practice and patient care. Study results are certainly not always translated into standards of care. Studies suggest that cancer care is uneven3. Variability in treatment recommendations and offerings exists among oncologists as well as institutionally at treatment centers.
Cancer Clinical Trials has information about the medical research process, including phases of clinical trials.
What are some physician perspectives about health care decision-making and evidence?
Richard Horton, MD, editor in chief of the Lancet, showcases how the precautionary principle is applied to the topic of very sick people needing to make timely treatment decisions in the absence of medical certainty and the dilemma physicians face on advising them.
“We (as physicians) must act on facts, and on the most accurate interpretations of them, using the best scientific information. That does not mean that we must sit back until we have 100% evidence about everything4.”
-Richard Horton, MD
David Katz, MD, Director and Founder of the Yale University’s Prevention Research Center, Director and Founder of the Integrative Medicine Center at Griffin Hospital, and Founder and President of Turn the Tide Foundation, refers to the challenges of health care decision making for chronic conditions in particular.
“Although the importance of scientific evidence in modern medicine is indisputable, its application is often questionable. Evidence simply does not exist to indicate the best treatments for many chronic conditions and syndromes. Under such circumstances, practitioners who choose to view evidence rigidly may have nothing to offer their patients but an apology5.”
-David L. Katz, MD